Medical Device Data System
Medical device data system. The foundation for such inter-communication is hardware and software typically referred to as medical device data systems MDDS that transfer. Medical devices like other computer systems can be vulnerable to security breaches potentially impacting the safety and effectiveness of the device. The study includes only those devices in the categories of anesthesiology cardiology diagnostics radiology general hospital use and surgery.
2 Characterization of the Data A medical device may be as simple as a tongue depressor but this paper is concerned only with those containing software. Medtronic has announced initial data from an early feasibility study to assess the Intrepid Transcatheter Mitral Valve Replacement TMVR system that utilises the new transfemoral delivery system. Furthermore submissions to the UDI database must also include Global Device Nomenclature GMDN codes and device registration holders will be required to include UDI numbers on post-market vigilance notifications involving their products.
A device that is intended to transfer store convert from one format to another according to preset specifications or display medical device data. The EU even determines the protocol https. According to Alan Cohen the director of systems engineering at Logic PD Android is a good operating system OS choice for some medical devices.
EUDAMED is the IT system developed by the European Commission to implement Regulation EU 2017745 on medical devices and Regulation EU 2017746 on in vitro diagnosis medical devices. Medical device makers often choose CE. An interface allowing on-line data entry by users an interface enabling the up-loading of XML files.
FDA has recently published a final rule for these type of systems fdagovNewsEventsNewsroomPressAnnouncementsucm243283htm but I have not found any relevant EU regulation for them. An MDDS acts only as the mechanism by which medical device data can be transferred stored converted or displayed. The new Regulations contain important improvements including a much larger EUDAMED database than the one that currently exists under the Medical Devices Directives.
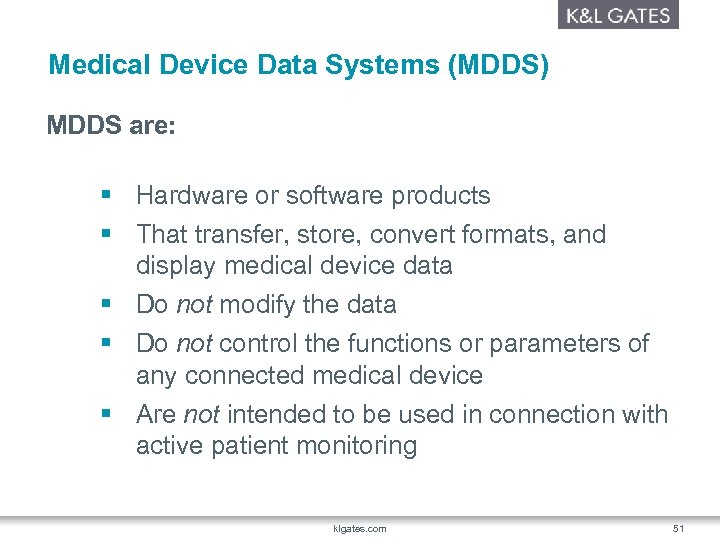
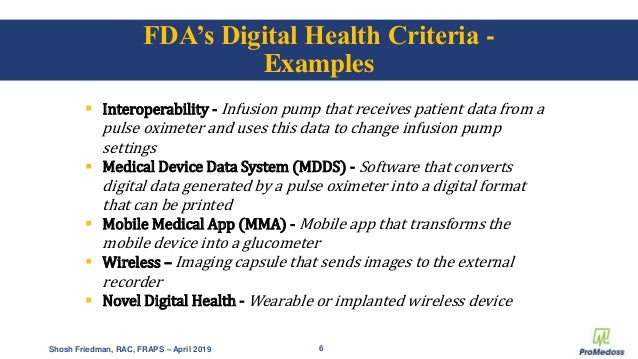
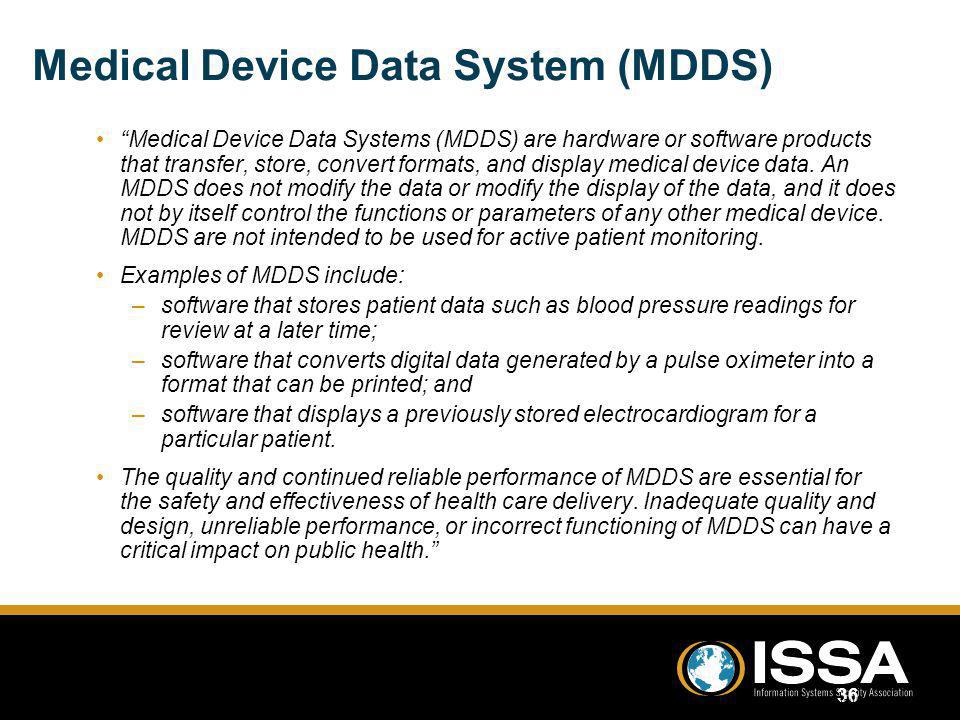
Medical Device Data Systems MDDS are hardware or software products intended to transfer store convert formats and display medical device data. A MDDS does not modify the data and it does not control the functions or parameters of any connected medical device. These systems mainly IT systems may provide electronic transfer storage and presentation of medical device and patient data.
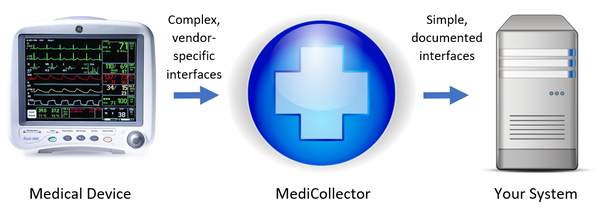
The US Food and Drug Administration FDA the government department that regulates the medical devices sector announced its intention to use ISO 13485 as the basis for its quality system legislation. Medical Device Data System MDDS MediCollector products operate as Medical Device Data Systems MDDS.
The US Food and Drug Administration FDA the government department that regulates the medical devices sector announced its intention to use ISO 13485 as the basis for its quality system legislation.
The foundation for such inter-communication is hardware and software typically referred to as medical device data systems MDDS that transfer. The electronic transfer of medical device data. Medical device makers often choose CE. An MDDS acts only as the mechanism by which medical device data can be transferred stored converted or displayed. The Global Unique Device Identification Database GUDID contains key device identification information submitted to the FDA about medical devices that have Unique Device Identifiers UDI. The electronic storage of medical device data. The main purpose of it is to provide a better monitoring of the data collected by the hardware devices while storing it for the future. An MDDS does not modify the data. The study includes only those devices in the categories of anesthesiology cardiology diagnostics radiology general hospital use and surgery.
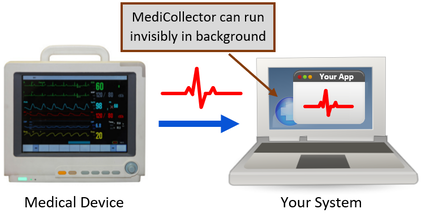
A medical device data system MDDS is a device that is intended to provide one or more of the following uses without controlling or altering the functions or parameters of any connected medical devices. According to Alan Cohen the director of systems engineering at Logic PD Android is a good operating system OS choice for some medical devices. Medtronic has announced initial data from an early feasibility study to assess the Intrepid Transcatheter Mitral Valve Replacement TMVR system that utilises the new transfemoral delivery system. The electronic storage of medical device data. A medical device CMS is the one that provides an easy integration matrix between a medical device hardware and a data management system software. On Friday Feb 6 2014 the FDA issued two final guidance documents that detail the agencys plans to loosen its regulation of medical device data systems and mobile applications Health Data Management reports. This means that our software acquires and transports data from medical devices but never alters or modifies the data nor does MediCollector interfere with the operation of the connected medical device.
































Post a Comment for "Medical Device Data System"